Available literature, largely consisting of observational studies, has explored the use of prothrombin complex concentrates (PCC) for coagulopathy due to various clinical scenarios other than oral anticoagulation, such as in liver disease, trauma, and cardiac surgery. Current evidence does not support the routine use of PCC in these scenarios as it has shown no definitive mortality benefit compared to non-PCC strategies (e.g., fresh frozen plasma [FPP]). Additionally, studies have shown mixed...
Several reviews and systematic analyses explore the use of prothrombin complex concentrates (PCC) in a variety of settings.
Chronic liver disease:
A 2023 review summarizes current evidence on the use of PCC in chronic liver disease (CLD), emphasizing that cirrhosis produces a fragile but “rebalanced” hemostatic state in which conventional tests such as international normalized ratio (INR) poorly reflect bleeding risk. In vitro studies consistently show that PCC markedly increases thrombin generation in cirrhotic plasma—more so than fresh frozen plasma (FFP)—and this effect becomes stronger with worsening liver disease, indicating potential utility but also raising concern for thrombotic complications. Clinical data remain limited and are largely retrospective, heterogeneous, and underpowered. Across studies of prophylactic use before procedures (e.g., TIPS, paracentesis, hepatobiliary interventions, or liver transplantation) PCC reliably improves INR but has inconsistent imp...
READ MORE→
A search of the published medical literature revealed
3 studies investigating the researchable question:
What is the literature supporting the use of prothrombin complex concentrate for coagulopathy not due to oral anticoagulant therapy?
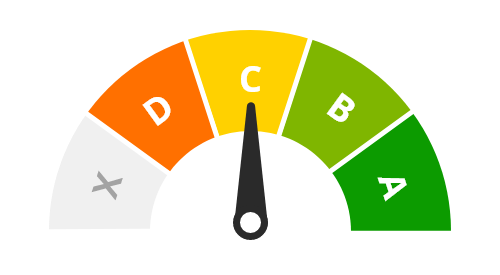
Level of evidence
C - Multiple studies with limitations or conflicting results
READ MORE→
[1] van Dievoet MA, Stephenne X, Rousseaux M, Lisman T, Hermans C, Deneys V. The use of prothrombin complex concentrate in chronic liver disease: A review of the literature. Transfus Med. 2023;33(3):205-212. doi:10.1111/tme.12969
[2] Kojundzic I, Alavi N, Lam A, et al. Use of prothrombin complex concentrates in liver transplantation: a systematic review and meta-analysis. Br J Anaesth. 2025;135(5):1172-1192. doi:10.1016/j.bja.2025.07.079
[3] Tanaka KA, Shettar S, Vandyck K, Shea SM, Abuelkasem E. Roles of Four-Factor Prothrombin Complex Concentrate in the Management of Critical Bleeding. ...