Current guideline recommendations and pooled comparative evidence support both misoprostol and dinoprostone as effective options for cervical ripening and labor induction in term, singleton pregnancies with intact membranes, with misoprostol administered orally or vaginally and dinoprostone available as a vaginal gel or sustained-release vaginal insert. Across multiple analyses, rates of vaginal delivery, cesarean delivery, and key neonatal outcomes such as low Apgar scores and neonatal inten...
A 2025 Clinical Practice Guideline from the American College of Obstetricians and Gynecologists (ACOG) extensively evaluates contemporary methods for cervical ripening in term, singleton, vertex pregnancies with intact membranes. The panel highlighted that commonly used pharmacologic agents for cervical ripening include misoprostol, a prostaglandin E1 analogue, and dinoprostone, a prostaglandin E2 preparation. Both are recommended options, with oral or vaginal misoprostol supported by strong recommendations and high quality evidence, and vaginal dinoprostone supported by strong recommendations with moderate quality evidence. Misoprostol may be administered orally or vaginally in a range of dosing regimens, whereas dinoprostone is available as a vaginal gel or a 10 mg vaginal insert. Misoprostol is contraindicated in patients with a history of uterine surgery, including cesarean delivery, because of the risk of uterine rupture, and selection of any agent should consider patient chara...
READ MORE→
A search of the published medical literature revealed
11 studies investigating the researchable question:
Please send us summary of studies/data comparing misoprostol vs Dinoprostone.
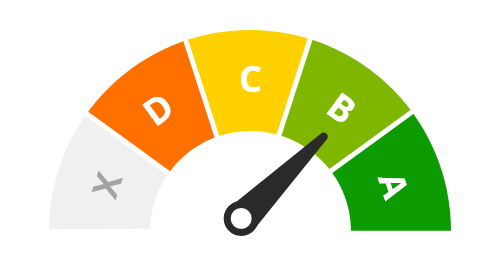
Level of evidence
B - One high-quality study or multiple studies with limitations
READ MORE→
[1] Cervical Ripening in Pregnancy: ACOG Clinical Practice Guideline No. 9. Obstet Gynecol. 2025;146(1):148-160. doi:10.1097/AOG.000000000000595
[2] World Health Organization. WHO Recommendations for Induction of Labour. Geneva: World Health Organization; 2011.
[3] World Health Organization. WHO Recommendations for Induction of Labour, At or Beyond Term. Geneva: World Health Organization; 2022.
[4] Yount SM, Lassiter N. The pharmacology of prostaglandins for induction of labor. J Midwifery Womens Health. 2013;58(2):133-44.
[5] Liu A, Lv J, Hu Y, Lang J, Ma L, Chen W. Efficacy and safety of intravaginal misoprostol versus intracervical dinoprostone for labor induction at term: a systematic review and meta-analysis. J Obstet Gynaecol Res. 2014;40(4):897-906.
[6]Tan SY, Au N, Peel MD, et al. Oral misoprostol (PGE1) vs vaginal dinoprostone (PGE2) for labor induction: individual participant data meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol. Published online November 8, 2025. doi:10.1002/uog.70100
[7] Lakho N, Hyder M, Ashraf T, et al. Efficacy and safety of misoprostol compared with dinoprostone for labor induction at term: an updated systematic review and meta-analysis of randomized controlled trials. Front Med (Lausanne). 2024;11:1459793. Published 2024 Dec 9. doi:10.3389/fmed.2024.1459793
[8] Ramadan M, Bashour G, Eldokmery E, et al. The efficacy and safety of oral and vaginal misoprostol versus dinoprostone on women experiencing labor: A systematic review and updated meta-analysis of 53 randomized controlled trials. Medicine (Baltimore). 2024;103(40):e39861. doi:10.1097/MD.0000000000039861
[9] Patabendige M, Chan F, Vayssiere C, et al. Vaginal misoprostol versus vaginal dinoprostone for cervical ripening and induction of labour: An individual participant data meta-analysis of randomised controlled trials. BJOG. 2024;131(9):1167-1180. doi:10.1111/1471-0528.17794
[10] Taliento C, Manservigi M, Tormen M, et al. Safety of misoprostol vs dinoprostone for induction of labor: A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2023;289:108-128. doi:10.1016/j.ejogrb.2023.08.382
[11] Wang L, Zheng J, Wang W, Fu J, Hou L. Efficacy and safety of misoprostol compared with the dinoprostone for labor induction at term: a meta-analysis. J Matern Fetal Neonatal Med. 2016;29(8):1297-1307. doi:10.3109/14767058.2015.1046828
[12] Austin SC, Sanchez-Ramos L, Adair CD. Labor induction with intravaginal misoprostol compared with the dinoprostone vaginal insert: a systematic review and metaanalysis. Am J Obstet Gynecol. 2010;202(6):624.e1-624.e6249. doi:10.1016/j.ajog.2010.03.014
[13] Facchinetti F, Fontanesi F, Del Giovane C. Pre-induction of labour: comparing dinoprostone vaginal insert to repeated prostaglandin administration: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2012;25(10):1965-1969. doi:10.3109/14767058.2012.668584