Available literature addressing extravasation of angiotensin II (Giapreza) is limited and largely extrapolated from class-based vasopressor management recommendations. Vasopressor extravasation is described as a vasoconstriction-mediated injury, with consistent recommendations for immediate infusion discontinuation, attempted aspiration of residual drug, catheter removal, limb elevation, and application of warm compresses. Pharmacologic management identifies subcutaneous phentolamine as first...
The 2023 focused update on management of noncytotoxic extravasation injuries provides an updated synthesis of treatment strategies for extravasation events, with particular emphasis on vasopressors and hypertonic saline, including peripheral administration considerations. Within this review, angiotensin II is discussed as part of the vasopressor class, and the authors explicitly state that data for the treatment of angiotensin II extravasations are limited. The review cites a single historical report published in 1963 describing angiotensin II–associated vascular effects, in which phentolamine was used successfully, although detailed extravasation management data are not available due to the age and limited accessibility of the report. Additionally, few angiotensin II extravasations have been described following peripheral, subcutaneous, or intradermal administration, with no cases resulting in local ischemic injury or necrosis; management therefore relies on class-level vasopressor...
READ MORE→
A search of the published medical literature revealed
2 studies investigating the researchable question:
What recommendations exist for managing extravasation of angiotensin II (Giapreza)?
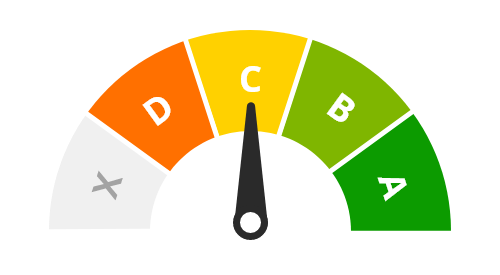
Level of evidence
C - Multiple studies with limitations or conflicting results
READ MORE→
[1] Stefanos SS, Kiser TH, MacLaren R, Mueller SW, Reynolds PM. Management of noncytotoxic extravasation injuries: A focused update on medications, treatment strategies, and peripheral administration of vasopressors and hypertonic saline. Pharmacotherapy. 2023;43(4):321-337. doi:10.1002/phar.2794
[2] Depasquale NP, Burch GE. Angiotensin II, digital blood flow, and the precapillary and postcapillary blood vessels of man. Ann Intern Med. 1963;58:278-292.
[3] Ong J, Van Gerpen R. Recommendations for Management of Noncytotoxic Vesicant Extravasations. J Infus Nurs. 2020;43(6):319-343. doi:10....